Learning Outcomes
By the end of this lesson, students will be able to:
i. Explain the rule of "like dissolves like," recognizing its significance in predicting the solubility of one substance in another.
ii. Differentiate between polar and nonpolar molecules, understanding the role of molecular polarity in solubility behavior.
iii. Apply the rule of "like dissolves like" to predict the solubility of various substances in different solvents, such as water, oil, and alcohol.
iv. Interpret solubility data and explain why certain substances dissolve in particular solvents while others do not.
v. Appreciate the practical applications of the rule of "like dissolves like" in various fields, including chemistry, environmental science, and everyday life.
Introduction
The rule of "like dissolves like" stands as a fundamental principle in chemistry, providing a simple yet effective way to predict the solubility of one substance in another. This rule states that polar substances dissolve in polar solvents, while nonpolar substances dissolve in nonpolar solvents. Understanding the concept of molecular polarity and its relationship to solubility is essential for comprehending various chemical processes and interpreting solubility observations.
i. Polar vs. Nonpolar Molecules: A Tale of Two Worlds
Polar molecules possess an uneven distribution of electrons, resulting in a partial positive charge on one end of the molecule and a partial negative charge on the other end. Water, with its bent molecular structure, is a classic example of a polar molecule. Nonpolar molecules, on the contrary, have a symmetrical distribution of electrons, lacking any significant partial charges. Oil, with its symmetrical molecular structure, is an example of a nonpolar molecule.
ii. "Like Dissolves Like": A Guiding Principle
The rule of "like dissolves like" stems from the attractive forces between molecules. Polar molecules attract each other through dipole-dipole interactions, while nonpolar molecules attract each other through weaker dispersion forces. Since polar molecules have similar charge distributions, they can interact favorably with each other, leading to dissolution in polar solvents. Similarly, nonpolar molecules, with their weak dispersion forces, tend to dissolve in nonpolar solvents due to similar intermolecular interactions.
iii. Predicting Solubility: Putting the Rule into Practice
Using the rule of "like dissolves like," we can predict the solubility of various substances in different solvents:
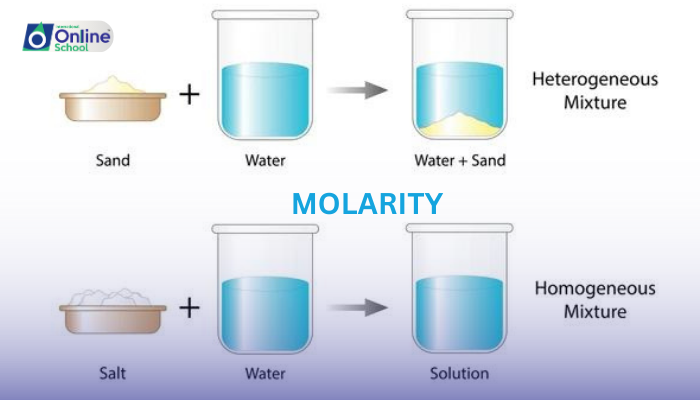
Polar Substances in Polar Solvents: Polar substances, such as table salt (NaCl) or sugar (C12H22O11), will dissolve in polar solvents like water, forming homogeneous solutions.
Nonpolar Substances in Nonpolar Solvents: Nonpolar substances, such as oil or wax, will dissolve in nonpolar solvents like oil or gasoline, forming homogeneous mixtures.
Polar and Nonpolar Substances Together: Polar substances will not dissolve in nonpolar solvents, and vice versa. For instance, oil will not dissolve in water, and salt will not dissolve in oil.
iv. Interpreting Solubility Data: Making Sense of Observations
Solubility data often provides insights into the polarity of substances and their interactions with solvents:
High Solubility: A high solubility of a substance in a particular solvent suggests that the solute and solvent have similar polarity, leading to favorable interactions and dissolution.
Low Solubility: Low solubility or insolubility indicates that the solute and solvent have different polarities, resulting in weak intermolecular forces and limited dissolution.
The rule of "like dissolves like" serves as a valuable tool for predicting solubility and understanding the behavior of substances in various solvents. By comprehending the concept of molecular polarity and its relationship to solubility, students gain a deeper appreciation for the intricacies of chemical interactions and the diverse phenomena observed in the world of solutions.